Microwave-driven carbonation of brucite
https://www.sciencedirect.com/science/article/pii/S2212982024000350
Marcello Campione, Mattia Corti, Daniela D’Alessio, Giancarlo Capitani, Andrea Lucotti, Rossella Yivlialin, Matteo Tommasini, Gianlorenzo Bussetti, Nadia Malaspina
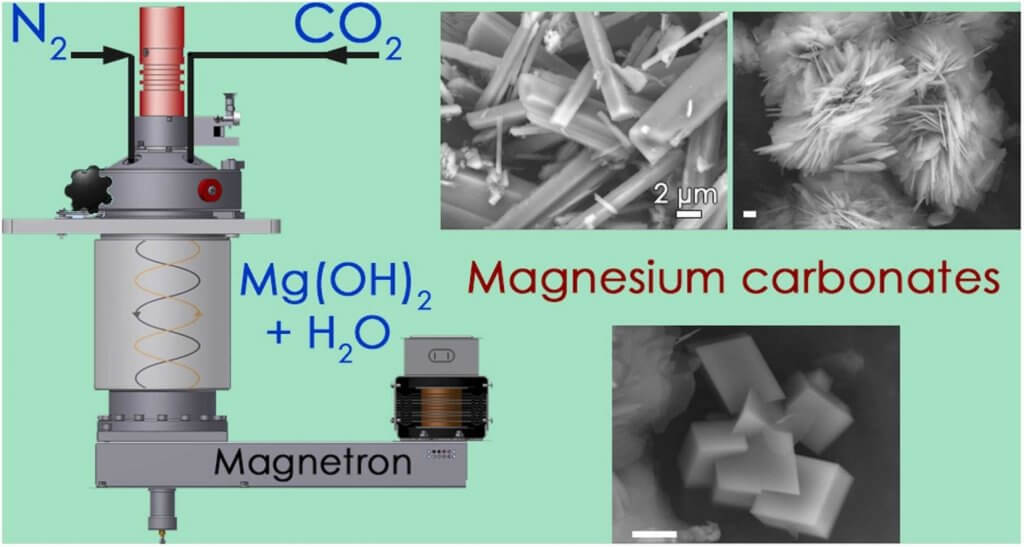
The water-mediated mineral carbonation represents a promising solution for the capture and the storage of atmospheric CO2. Even though this reaction might be spontaneous for a number of Mg- and Ca-rich mineral phases, it is characterized by considerable activation barriers. In order to make it effective, associated energy costs related to the achievement of adequate reaction conditions must be minimized. Microwave chemistry is known to provide for substantial increments of the reaction rate for several systems. We applied here microwave chemistry to the process of carbonation of aqueous slurries of brucite, a model system of Mg-rich mineral, subjected to partial pressures of CO2 as low as 6 bar and to no other additive. The temperature of the reactor was finely varied while the radiation power and the reactor pressure were monitored in real-time. The radiation power was used to estimate the radiation energy budget needed to complete the carbonation process, whereas the reactor pressure was used as a proxy of reaction progression. We show a detailed evolution of the carbonate products obtained in terms of mineral phases, morphological properties, and degree of crystallinity, both as precipitate and as solid residue in the exsiccated supernatant reaction liquid.